Controversial terms
Collision Induced Dissociation vs. Collisionally Activated Dissociation
Should CAD be replaced in all cases by CID?
In the literature
A search for occurrences (in 2005) of the two terms in the literature reveals a distinct preference.
Collision-induced dissociation (CID) and collisionally activated dissociation (CAD) refer to the process in which a collision between and ion and a neutral species results in the conversion of part of the translational energy into internal energy of the ion and subsequent fragmentation. The IUPAC document defines the two terms equivalently as does Price (JASMS, 2, 336, 1991). The ASMS Terms and Definitions document does not mention CAD. Sparkman defines CAD and CID equivalently, but notes his preference for CAD.
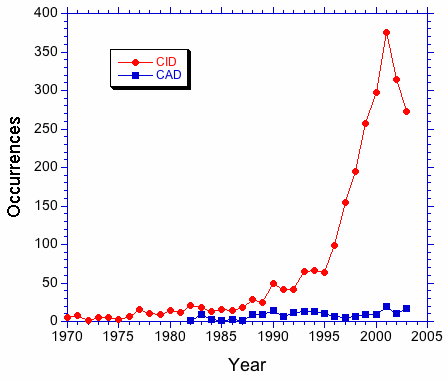
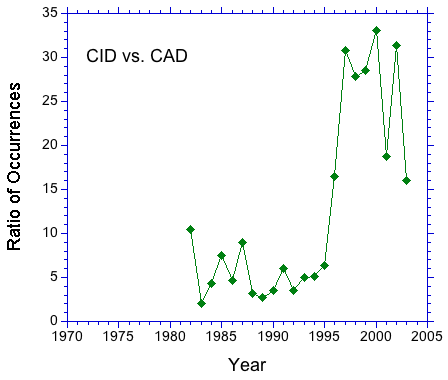
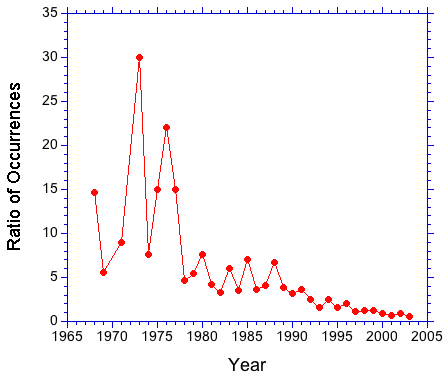
A search of the literature for "collision induced dissociation" and "collisionally activated dissociation" suggests that the former term is preferred. In Figure 1, the number of occurrences of the above strings in journal articles is plotted as a function of the year of publication. The plot shows a clear preference for CID over CAD that increases after 1990. This trend can be seen clearly in Figure 2. The occurrence ratio is about 5 in the 80s and early 90s, then jumps to about 30 in the late 90s.
Based on this data, should the IUPAC document list collision induced dissociation/CID as the preferred term?
Google fight
Which is the more widely used term per Google: collision induced dissociation vs collisionally activated dissociation (CID usually wins).
Mass Resolution vs. Mass Resolving Power
How should Resolution and Resolving Power be defined?
| IUPAC RECOMMENDATIONS 2013 |
| Controversial terms |
|---|
In a mass spectrum, the observed m/z value divided by the smallest difference Δ(m/z) for two ions that can be separated: (m/z)/Δ(m/z).
|
| Related Term(s): Resolving power |
| Reference(s):
IUPAC. Analytical Division. Compendium of Analytical Nomenclature (the Orange Book). Definitive Rules, 1979. Compiled by J. Inczdy, T. Lengyel, A. M. Ure. Blackwell Scientific Publications, Oxford (1997). On-line corrected version: http://www.iupac.org /publications/analytical compendium (2000). IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. G. L. Glish, D. J. Burinsky. J. Am. Soc. Mass Spectrom. 19, 161 (2008). J. Laskin, J. H. Futrell. Mass Spectrom. Rev. 24, 135 (2005). |
| From Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations 2013); DOI: 10.1351/PAC-REC-06-04-06 © IUPAC 2013. |
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| Controversial terms |
|---|
|
resolution in mass spectroscopy [sic] (energy): By analogy with the peak width definition for mass resolution, a peak showing the number of ions as a function of their translational energy should be used to give a value for the energy resolution. (10 per cent valley definition): Let two peaks of equal height in a mass spectrum at masses m and m - Δm be separated by a valley which at its lowest point is just 10 per cent of the height of either peak. For similar peaks at a mass exceeding m, let the height of the valley at its lowest point be more (by any amount) than ten per cent of either peak height. Then the resolution (10 per cent valley definition) is m/Δm. It is usually a function of m. The ratio m/Δm should be given for a number of values of . (peak width definition): For a single peak made up of singly charged ions at mass in a mass spectrum, the resolution may be expressed as m/Δm where Δm is the width of the peak at a height which is a specified fraction of the maximum peak height. It is recommended that one of three values 50%, 5% or 0.5% should always be used. For an isolated symmetrical peak recorded with a system which is linear in the range between 5% and 10% levels of the peak, the 5% peak width definition is technically equivalent to the 10% valley definition. A common standard is the definition of resolution based upon being Full Width of the peak at Half its Maximum height, sometimes abbreviated 'FWHM'. This acronym should preferably be defined the first time it is used. Source: PAC, 1991, 63, 1541 (Recommendations for nomenclature and symbolism for mass spectroscopy (including an appendix of terms used in vacuum technology). (Recommendations 1991)) on page 1554 Orange Book, p. 203 |
| IUPAC Gold Book |
| Index of Gold Book Terms |
Orange Book
| ORANGE BOOK DEFINITION
IUPAC. Analytical Division. Compendium of Analytical Nomenclature (the Orange Book). Definitive Rules, 1979 (see also Orange Book 2023) |
| Controversial terms |
|---|
|
Resolution: 10 Per Cent Valley Definition Let two peaks of equal height in a mass spectrum at masses m and m - Δm be separated by a valley which at its lowest point is just 10% of the height of either peak. For similar peaks at a mass exceeding m , let the height of the valley at its lowest point be more (by any amount) than 10% of either peak. Then the resolution (10% valley definition) is m / Δm. The ratio m /Δm should be given for a number of values of m. Resolution: Peak Width Definition For a single peak made up of singly charged ions at mass m in a mass spectrum, the resolution may be expressed as m / Δm, where Δm is the width of the peak at a height which is a specified fraction of the maximum peak height. It is recommended that one of three values 50%, 5% or 0.5% should always be used. (Note that for an isolated symmetrical peak recorded with a system which is linear in the range between 5% and 10% levels of the peak, the 5% peak width definition is equivalent to the 10% valley definition. A common standard is the definition of resolution based upon Δm being Full Width of the peak at Half its Maximum (FWHM) height. |
| IUPAC 1997 Orange Book Chapter 12 |
| Index of Orange Book Terms |
Resolution and resolving power controversy
| QUOTED TEXT FROM IUPAC RECOMMENDATIONS 2013 |
| The IUPAC definition of resolution in mass spectrometry expresses this value as m/Δm, where m is the mass of the ion of interest and Δm is the peak width (peak width definition) or the spacing between two equal intensity peaks with a valley between them no more than 10 % of their height (10 % valley definition) [1]. Resolving power in mass spectrometry is defined as the ability of an instrument or measurement procedure to distinguish between two peaks at m/z values differing by a small amount and expressed as the peak width in mass units [2]. Mass resolving power is defined separately as m/Δm in a manner similar to that given above for mass resolution [3]. These definitions of mass resolving power and resolving power in mass spectrometry are contradictory, the former is expressed as a dimensionless ratio and the latter as a mass. The definitions for resolution in mass spectrometry and resolving power in mass spectrometry come from Todd's 1991 recommendations [4], and the definition for mass resolving power comes from Beynon's 1978 recommendations [5]. Beynon's work contains no definition for mass resolution.
Alternative definitions for resolution and resolving power in mass spectrometry have been proposed [6][7]. It has been suggested that resolution be given by Δm and resolving power by m/Δm; however, these definitions are not widely used. The majority of the mass spectrometry community uses resolution as defined by IUPAC. The term resolving power is not widely used as a synonym for resolution. In this document, the IUPAC definition of resolution in mass spectrometry remains in place. The definition of resolving power has been adapted from the current IUPAC definition of mass resolving power. |
| From Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations 2013); DOI: 10.1351/PAC-REC-06-04-06 © IUPAC 2013. |
|---|
Recent article
- Murray, K. K. Resolution and Resolving Power in Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2022. http://dx.doi.org/10.1021/jasms.2c00216 See also[8]
Examples of use
Books defining resolution and/or resolving power
Books using resolution is m/Δm
- Mass Spectrometry and its Applications to Organic Chemistry
- J. H. Beynon, Elsevier, 1960
- p. 51 "The terms 'resolution' and 'resolving power' have been used a great deal in the above discussion. It has been assumed that the doublet is 'resolved' when its constituent ion species are 'separated' and that the difficult of separation or 'resolving power' necessary to separate the adjacent mass peaks is given by M/ΔM."
- Mass Spectrometry - Organic Chemical Applications
- Klaus Biemann, McGraw-Hill, 1962
- (p. 13) the term resolution is used in different ways - Throughout this book resolution will be considered as M/ΔM
- Lasers and Mass Spectrometry
- By David M. Lubman, Oxford University Press US, 1990, ISBN 0195059298
- Interpretation of Mass Spectra
- Fred W. McLafferty, Turecek, University Science Books, 1993, Language: English, ISBN 0935702253
- Mass Spectrometry: Clinical and Biomedical Applications
- By Dominic M. Desiderio, Springer, 1993, ISBN 0306442612
- Practical Organic Mass Spectrometry: A Guide for Chemical and Biochemical Analysis
- J. R. Chapman, Wiley_Default, 1995, ISBN 047195831X
- Mass Spectrometry for Chemists and Biochemists
- Robert Alexander Walker Johnstone, M. E. Rose, Cambridge University Press, 1996, ISBN 0521424976
- Introduction to Mass Spectrometry
- By J. Throck Watson, Lippincott-Raven, 1997, ISBN 0397516886
- Ionization Methods in Organic Mass Spectrometry
- By Alison E. Ashcroft, Royal Society of Chemistry (Great Britain), Royal Society of Chemistry, 1997, ISBN 0854045708
- Accelerator Mass Spectrometry: Ultrasensitive Analysis for Global Science
- Claudio Tuniz, John R. Bird, Gregory F. Herzog, David Fink, CRC Press, 1998, ISBN 0849345383
- Mass Spectrometry in Biology & Medicine
- By A. L. Burlingame, Steven A. Carr, Michael A. Baldwin, Humana Press, 1999, ISBN 0896037991
- Mass Spectrometry and Genomic Analysis
- J. Nicholas Housby, Springer, 2001, ISBN 0792371739
- Mass Spectrometry Basics
- Christopher G. Herbert, Robert Alexander Walker Johnstone, CRC Press, 2002, ISBN 0849313546
- Liquid Chromatography Mass Spectrometry: An Introduction Robert E. Ardrey
- Wiley, 2003, ISBN 0471498017
- Mass Spectrometry: A Textbook
- Jurgen H. Gross, Springer, 2004, ISBN 3540407391
- Quadrupole Ion Trap Mass Spectrometry
- By Raymond E. March, John F. Todd, Wiley-IEEE, 2005, ISBN 0471717975
- The Expanding Role of Mass Spectrometry in Biotechnology
- Gary Siuzdak, McC Pr, 2006, ISBN 0974245127
- Quantitative Applications of Mass Spectrometry
- Pietro Traldi, Franco Magno, Irma Lavagnini, Roberta Seraglia, Wiley, 2006, ISBN 0470025166
- Assigning Structures to Ions in Mass Spectrometry
- John L. Holmes, Christiane Aubry, Paul M. Mayer, CRC, 2006, ISBN 0849319501
- Mass Spectrometry: Principles and Applications
- Edmond de Hoffmann, Vincent Stroobant, Wiley-Interscience, 2007
- ISBN 047003310X
- Mass Spectrometry: Principles and Applications
- Edmond de Hoffmann, Jean Charette, Vincent Stroobant, Wiley, 1996, ISBN 0471966975
- p 287: "Resolution: the ratio of m/δm where m and m+δm are the mass numbers of the two ions that yield neighboring peaks with a valley depth of x% of the weakest peak's intensity."
- Quantitative Proteomics by Mass Spectrometry (Methods in Molecular Biology)
- Salvatore Sechi, Humana Press, 2007, ISBN 1588295710
- Computational Methods for Mass Spectrometry Proteomics
- Ingvar Eidhammer, Kristian Flikka, Lennart Martens, Svein-Ole Mikalsen, Wiley-Interscience, 2008, ISBN 0470512970
Books that use resolution is Δm
- Mass Spectrometry Desk Reference
- David Sparkman, Global View, 2006, ISBN 0966081390
- "Incorrect: resolution - when defined in the same way as resolving power. Resolution is the inverse of resolving power and expressed as ΔM at M."
- Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation
- J. Throck Watson, O. David Sparkman, Wiley, 2007, Language: English, ISBN 0470516348
- Fundamentals of Contemporary Mass Spectrometry
- Chhabil Dass, 2007, ISBN 0471682292
- p. 68: "[mass resolution] is the inverse of resolving power (RP), given as RP=m/Δm"
- Proteomics in Practice: A Guide to Successful Experimental Design
- Reiner Westermeier, Tom Naven, Hans-Rudolf H??pker, Wiley, 2008, ISBN 3527319417
Early manuscripts defining resolution and/or resolving power
- F. Aston, Bakerian lecture. "A new mass-spectrograph and the whole number rule", Proc. R. Soc. London, 1927. http://dx.doi.org/10.1098/rspa.1927.0106
- "Its resolving power was sufficient to separate mass lines differing by about 1 in 130 and its accuracy of measurement was about 1 in 1000. [] It was finally decided that the increase of resolution could best be obtained by doubling the angles of electric and magnetic deflection, and sharpening the lines by the use of finer slits placed further apart, in addition special methods were considered for the necessary increase of accuracy in measurement. After numerous setbacks all these objects have been successfully carried out. The new instrument has five times the resolving power of the old one, far more than sufficient to separate the mass lines of the heaviest element known. Its accuracy is 1 in 10,000 which is just sufficient to give rough first order values of the divergences from whole numbers."
- F.W. Aston, "Atoms and their Packing Fractions", Nature, 120 (1927) 956-959. http://dx.doi.org/10.1038/120956a0
- "the resolution of the mass lines of the heavier elements [] resolving power was suflicient to separate mass lines differing by about 1 in 130, and its accuracy of measurement was about 1 in 1000. [] new instrument has five times the resolving power of the old one, far more than sufficient to separate the mass lines of the heaviest element known. Its accuracy is 1 in 10,000, []"
- W. Bleakney, "A New Method of Positive Ray Analysis and Its Application to the Measurement of Ionization Potentials in Mercury Vapor," Phys. Rev., 34 (1929) 157-160. http://dx.doi.org/10.1103/PhysRev.34.157
- "While the resolving power of the analyzer is not particularly high, yet it has proved to be excellent for the purposes for which it was designed."
- F.W. Aston, The Isotopic Constitution and Atomic Weight of Lead from Different Sources, Proceedings of the Royal Society of London Series a-Containing Papers of a Mathematical and Physical Character, 140 (1933) 535-543. http://dx.doi.org/10.1098/rspa.1933.0087
- "a view to increasing resolving power [] Increased accuracy has been obtained, but full advantage cannot be taken of it until higher resolution is available on account of the inevitable error involved in measuring the distance between lines not of the same intensity"
- A.J. Dempster, New Methods in Mass Spectroscopy, Proceedings of the American Philosophical Society, 75 (1935) 755-767. http://dx.doi.org/
- "The main limitation to increased accuracy in mass determinations is in the comparatively small resolving power of the mass spectrographs hitherto used. On page 78 of "Mass Spectra and Isotopes," Aston says: "The resolving power is sufficient to separate lines differing by I in 6oo, . . . since the lines are irregularly curved and change in shape as one moves from one end of the spectrum to the other, it is impossible to assign positions to them relative to the fiducial spot with sufficient accuracy to approach the figure of 1 in 10,000 aimed at. This can only be done by measuring the distance between lines of approximately the same intensity and therefore the same shape, when they are quite [] The spectra reproduced by Bainbridge 1 show a resolving power of approximately 1 in 200, that is, the image produced by the atoms of one element is so broad that the value obtained for the weight, if one side of the image is observed, differs by 1 in 200 from the weight obtained if the other side is used. Of course, the center is measured but some of the mass determinations given by Dr. Bainbridge involve estimating the center of the image with an accuracy of one hundredth of the width of the image. While the progress made by Dr. Aston and Dr. Bainbridge has been most re- markable, it is permissible to hope that an increase in sharpness of the images with a corresponding increase in resolving power would give a still greater precision in atomic mass determinations.close together. The accuracy of 1 in 1O,OOO estimated by Dr. Aston implies the judging of the centers, [] As explained in the introduction, this is primarily a problem of increased resolution with greater sharpness of the ion images. [] The resolving power with this comparatively wide slit is I in 1OOO."
Other IUPAC definitions of resolution
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| Controversial terms |
|---|
|
http://goldbook.iupac.org/R05319.html resolution (in optical spectroscopy) Wavenumber, wavelength or frequency difference of two still distinguishable lines in a spectrum. Source: Green Book, 2nd ed., p. 31
|
| IUPAC Gold Book |
| Index of Gold Book Terms |
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| Controversial terms |
|---|
| http://goldbook.iupac.org/P04465.html
peak resolution, Rs (in chromatography) The separation of two peaks in terms of their average peak width at base (t R2 > t R1):
In the case of two adjacent peaks it may be assumed that w b1 ? w b2, and thus, the width of the second peak may be substituted for the average value:
Source: PAC, 1993, 65, 819 (Nomenclature for chromatography (IUPAC Recommendations 1993)) on page 847 Orange Book, p. 108 |
| IUPAC Gold Book |
| Index of Gold Book Terms |
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| Controversial terms |
|---|
|
resolution (in gas chromatography) http://goldbook.iupac.org/R05317.html A characteristic of the separation of two adjacent peaks. It may be expressed according to the equation:
where RAB is the resolution, dR (A) and dR (B) are the retention distances (time or volume) of each eluted component A and B, and wA and wB are the respective widths of each peak at its base. PAC, 1990, 62, 2167 (Glossary of atmospheric chemistry terms (Recommendations 1990)) on page 2211 |
| IUPAC Gold Book |
| Index of Gold Book Terms |
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| Controversial terms |
|---|
| http://goldbook.iupac.org/E02113.html
energy resolution (in radiochemistry) A measurement, at given energy, of the smallest difference between the energies of two particles or photons capable of being distinguished by a radiation spectrometer. Source: PAC, 1994, 66, 2513 (Nomenclature for radioanalytical chemistry (IUPAC Recommendations 1994)) on page 2519 |
| IUPAC Gold Book |
| Index of Gold Book Terms |
Resolution and resolving power terminology in mass spectrometry
- ASMS 2022
- Poster MP 113
- Kermit K Murray
Premise
Nomenclature inconsistencies and conflicts can best be resolved through a detailed understanding of the origin and development of terms. The goal of this project is to investigate the origins and use as well as prior and current definitions of resolution and resolving power in order to make informed recommendations on the controversial and in some cases conflicting terminology.
Current definitions
In mass spectrometry, two peaks in a mass spectrum are resolved if they are distinguishable as separate. The degree to which the peaks are resolved can be quantified using the peak width or the separation between two peaks and is represented by (m/z) where m/z is the mass-to-charge ratio. For singly charged ions, this can be expressed as m or, in older publications, as M. The smallest value of m for which peaks are resolved is the limit of resolution. There are two general methods to determine m: peak width and valley:
Peak width: m is the peak width at a specified fraction of the peak height, for example at 50% m is the full width at half maximum
Valley: m is the separation between two equal height peaks that produces a valley a specified fraction of the height, for example 10%.
The 10% valley m is comparable to the 5% peak height m and approximately half that obtained from the FWHM. There are three general interpretations of the definitions of resolution and resolving power:
- the terms are equivalent and represented by m/m (Meyerson 1975, Murray 2013)
- resolution is m/m and resolving power is m (Price 1991, Todd 1991)
- resolving power is m/m and resolution is m (Beynon 1978).
Historical use
Prior to the Second World War, the term resolving power, defined as M/M, was used almost exclusively. Resolution was used as a binary variable or as the limit of resolution. In the second half of the 20th century, the two terms were increasingly used interchangeably.
F.W. Aston used resolution as a binary variable and resolving power as a quantitative measure, for example, the instrument will resolve beams of different masses if the change in for change of mass is greater than the geometrical spread, and the greater for a given mass and given spread the greater the resolving power (Aston 1922). In his book Mass Spectra and Isotopes, Aston defines resolving power as M/M (Aston 1933).
A. J. Dempster defined limit of resolution as m/m (Dempster 1918) and, like Aston, often used the construct one in [mass] for resolving power, as in resolving power with this comparatively wide slit is 1 in 1000 (Dempster 1935).
K. T. Bainbridge stated that resolving power is defined as the ratio M/M for complete separation of two lines and so is more stringent than the optical definition (Bainbridge 1936).
J. Mattauch defined resolving power as M/M and resolution as M/M (Mattauch 1936)
W. Bleakney used the term resolving power in a 1929 publication (Bleakney 1929) but defined resolution as m/m in a 1949 publication (Mariner 1949).
A. O. Nier used both resolving power (Nier 1936) as well as resolution (Nier 1960).
J. H. Beynon in his textbook Mass Spectrometry and its Applications to Organic Chemistry writes resolution and resolving power have been used a great deal in the above discussion. It has been assumed that the doublet is resolved when its constituent ion species are separated and that the difficult of separation or resolving power necessary to separate the adjacent mass peaks is given by M/M (Beynon 1960)
K. Biemann in his textbook Mass Spectrometry: Organic Chemical Applications, states that the term resolution is used in different ways Throughout this book resolution will be considered as M/M (Biemann 1962).
ASMS Definitions
Subcommittee 10 on Definitions and Terms of ASTM Committee E-14 on Mass Spectrometry was established in 1970 and presented a compendia of terms at the 1974 ASMS meeting (Meyerson 1975). The ASMS Nomenclature Committee presented a list of terms at the 1982 ASMS meeting in Honolulu (Cameron 1982) and terms assembled by the ASMS Measurements and Standards Committee were published in 1991 (Price 1991) which closely paralleled the contemporary IUPAC recommendations (Todd 1991).
IUPAC Definitions
There have been four IUPAC recommendations for mass spectrometry terminology in the past five decades produced by the IUPAC Analytical Chemistry Division Commission on Analytical Nomenclature (Robertson 1974), the IUPAC Physical Chemistry Division Commission on Molecular Structure and Spectroscopy (Beynon 1978), the IUPAC Physical Chemistry Division Commission on Molecular Structure and Spectroscopy Subcommittee on Mass Spectroscopy (Todd 1991), and the IUPAC Physical and Biophysical Chemistry Division (Murray 2013). The IUPAC Compendium of Chemical Terminology Gold Book gives definitions of resolution (valley and width) from Todd 1991 and gives two conflicting definitions for resolving power, one from Todd 1991 (also Robertson 1974) that defines resolving power as m and one from Beynon 1978 that defines resolving power as m/m.
Recommendations
Terminology recommendations for resolution and resolving power must take into account the current interchangeable use of the terms as well as the longstanding use of resolving power as m/m. It is the opinion of the author that resolution should be used as a binary variable, resolving power defined as m/m be encouraged, and limit of resolution defined as m/m be used where necessary.
Resolution: The use of resolution as a quantitative measure is discouraged: use resolving power or limit of resolution as appropriate.
Resolving power: The observed m/z value divided by the smallest difference (m/z) for two peaks that can be separated: (m/z)/(m/z).
Limit of resolution: The smallest difference (m/z) for two peaks that can be separated divided by m/z: (m/z)/(m/z).
The recommendations above are those of the author who hopes that these concepts will be considered when developing the next list of terminology.
References
Aston, F.W.: Some problems of the mass-spectrograph. Philos. Mag. 43, 514 (1922)
Aston, F.W.: Mass Spectra and Isotopes, Arnold, London, (1933).
Bainbridge, K.T., Jordan, E.B.: Mass Spectrum Analysis. Phys. Rev. 50, 282 (1936)
Biemann, K: Mass Spectrometry: Organic Chemical Applications, McGraw-Hill, New York (1962).
Bleakney, W.: A New Method of Positive Ray Analysis and Its Application to the Measurement of Ionization Potentials in Mercury Vapor. Phys. Rev. 34, 157 (1929)
Beynon, J.H.: Recommendations for Symbolism and Nomenclature for Mass Spectroscopy. Pure Appl. Chem. 50, 65 (1978)
Beynon, J.H. Mass Spectrometry and its Applications to Organic Chemistry, Elsevier, (1960)
Cameron, D.: ASMS Nomenclature Committee Workshop. Annual Conference on Mass Spectrometry and Allied Topics Abstracts. 30, 901 (1982).
Dempster, A.J.: A new method of positive ray analysis. Phys. Rev. 11, 316 (1918)
Dempster, A.J.: New Methods in Mass Spectroscopy. Proc, Am. Phil. Soc. 75, 755 (1935)
Mariner, T., Bleakney, W.: A large mass spectrometer employing crossed electric and magnetic fields. Rev. Sci. Instrum. 20, 297 (1949)
Meyerson, S.: Definitions and terms in mass spectrometry. Biomed. Mass Spectrom. 2, 59 (1975)
Mattauch, J.: A Double-Focusing Mass Spectrograph and the Masses of N15 and 018. Phys. Rev. 50, 617 (1936)
Murray, K.K., Boyd, R.K., Eberlin, M.N., Langley, G.J., Li, L., Naito, Y.: Definitions of terms relating to mass spectrometry, Pure. Appl. Chem. 85, 1515-1609 (2013)
Nier, A.O.: A Mass-Spectrographic Study of the Isotopes of Argon, Potassium, Rubidium, Zinc and Cadmium. Phys. Rev. 50, 1041 (1936)
Nier, A.O.: Small General Purpose Double Focusing Mass Spectrometer. Rev. Sci. Instrum. 31, 1127 (1960)
Price, P.: Standard definitions of terms relating to mass spectrometry. J. Am. Soc. Mass Spectrom. 2, 336 (1991)
Robertson, A.J.B.: Recommendations for Nomenclature of Mass Spectrometry. Pure Appl. Chem. 37, 469 (1974)
Todd, J.F.J.: Recommendations for Nomenclature and Symbolism for Mass-Spectroscopy. Pure. Appl. Chem. 63, 1541 (1991)
External links
- Urban, J., Afseth, N.K., tys, D.: Fundamental definitions and confusions in mass spectrometry about mass assignment, centroiding and resolution. TrAC Trends in Analytical Chemistry. 53, 126-136 (2014); http://dx.doi.org/10.1016/j.trac.2013.07.010
Mass-to-Charge Ratio
Should the Thomson be used instead of m/z?
Should be m/z be replaced by m/q?
Parent/Daughter vs. Precursor/Product
Should Parent Ion/Daughter Ion be replaced with Precursor Ion/Product Ion? How about nth generation products?
Statistics on Parent-Daughter vs. Precursor-Product
Here are some statistics (from 2005) on the Parent vs. Precursor and Daughter vs. Product debate.
A little more than a dozen years ago, it was suggested that the terms Parent Ion and Daughter Ion be replaced with Precursor Ion and Product Ion, respectively (see Glish, J. Am Soc. Mass Spectrom, 2, 349, 1991). The rationale is to avoid gender-specific terms to describe inanimate objects.
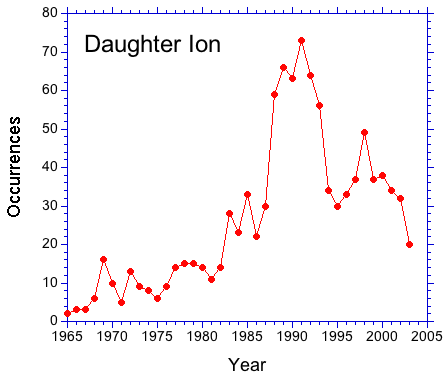
A check of the literature suggests that a shift in usage has in fact occurred. In the figure below, the occurrence of Daughter Ion is plotted as a function of year. The number of occurrences has dropped by about one-half since the early 90s. Quantifying the occurrences of Product Ion is difficult since the phrase yields results that are not related to mass spectrometry.
The plot below show the occurrences of precursor ion and parent ion. From this plot, it appears that the former term is now being used more frequently in place of the latter.
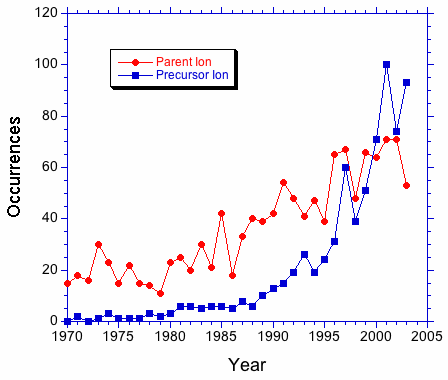
A plot of the ratio of occurrences seems to drive home this point.
A remaining issue is the nomenclature for nth generation product ions. Glish suggests x generation product ions where x=n-1 for a MSn experiment.
Slashes and Hyphens
How should Slashes and Hyphens be used in combined techniques?
- For specific usage, see the list of acronyms
Slashes or hyphens for combined methods
There is a great deal of confusion on the use of slashes, hyphens, spaces, or no spaces to indicate the combination of techniques, particularly when acronyms and abbreviations are used. The Chicago Manual of Style tends to favor hyphens due to the ambiguity of the slash, which has connotations of "and/or" in many instances. The ACS Style Guide makes no specific recommendations but gives examples of slashes, hyphens, spaces and no spaces in examples. The American Institute of Physics Style Manual makes no specific recommendation but contains no examples of the slash usage. David Sparkman calls for separate connotations of the slash and hyphen with the former separating techniques and the latter instruments. Rapid Communications in Mass Spectrometry has called for a slash to separate combined methods and a hyphen to highlight a particular component such as the ionization method (Sparkman instead suggests a space to separate the ionization method). The Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations 2013) suggests the use of the hyphen but indicates that the slash can also be used.
| QUOTED TEXT FROM IUPAC RECOMMENDATIONS 2013 |
| The hyphen, or alternatively the slash (forward stroke), can be used to indicate combined methods such as gas chromatography separation combined with mass spectrometry detection. Thus, the above combination can be written as gas chromatography-mass spectrometry or alternatively as gas chromatography/mass spectrometry. The corresponding abbreviations are GC-MS or GC/MS. The first use of a hyphen to indicate the combination of a separation method with mass spectrometry was in the early 1960s [9], and the use of a slash separator was in the 1970s [10]. The term hyphenated techniques was coined in 1980 [11]. Currently, hyphens and slashes are used interchangeably [12]. The journal Rapid Communications in Mass Spectrometry has in the past recommended that the combination of two analytical techniques be designated by a slash (Conventions adopted by RCM in Advice to Authors. Rapid Commun. Mass Spectrom. 17, Issue 1 (2003)). A recent Journal of Chromatography glossary also favors this usage [13]. IUPAC recommends that hyphens be used to describe variants of separation techniques, for example, gas-liquid chromatography and pyrolysis-gas chromatography [14]. The authors of this document are evenly split in their preference for hyphen or slash. For consistency with the prior recommendations, we use the hyphen for combined techniques but note that the slash can be used interchangeably. |
| From Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations 2013); DOI: 10.1351/PAC-REC-06-04-06 © IUPAC 2013. |
|---|
Other recommendations are given below.
Chicago Manual of Style
See http://www.chicagomanualofstyle.org/
The 16th edition of the Chicago Manual of Style indicates that slashes are most commonly used to indicate alternatives in the "and/or" formulation, for example "Hercules/Heracles."(CMOS 6.104) The CMOS also indicates that the slash is occasionally use to indicate "and" as in "Jekyll/Hyde." The "per" and "divided" by meanings are also noted.
The CMOS big table of hyphenation rules states that two nouns indicating two functions (the first noun doesn't modify the second) are hyphenated in both the noun and adjective forms.(CMOS 7.85)
American Chemical Society Style Guide
Chapter 10 of the ACS Style Guide[15] discusses editorial style including the use of hyphens and abbreviations.
Specific rules for combined methods are not given, but there are several examples in a list of abbreviations use space, no space, hyphen, en-dash, or slash. Surprisingly, neither GC-MS nor LC-MS are given in the list. Hyphen proponents will point to CE-MS, but slash advocates will point to CP/MAS.
Specific examples are: capillary electrophoresis mass spectrometry is abbreviated CE-MS, but cross-polarization/magic-angle spinning is abbreviated CP/MAS, but also CP-MAS, CP-MAS, CPMAS, and CP MAS are also indicated. Other examples are fast atom bombardment mass spectrometry (FABMS), Fourier transform ion cyclotron resonance (FTICR), Fourier transform infrared (FTIR, FT/IR, FT-IR, and FT IR), glow discharge mass spectrometry (GDMS), high-resolution mass spectrometry (HRMS), isotope dilution mass spectrometry (IDMS), isotopic ratio mass spectrometry (IRMS), laser desorption mass spectrometry (LDMS), matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS and MALDI-TOF MS), plasma desorption mass spectrometry (PDMS), pyrolysis-gas chromatography-mass spectrometry (Py-GC-MS), time-of-flight mass spectrometry (TOFMS TOF MS), triple-quadrupole mass spectrometry (TQMS).
American Institute of Physics Style Manual
The AIP style manual uses the hyphen exclusively for combined terms.[16]
Mass Spectrometry Desk Reference
David Sparkman in his Mass Spectrometry Desk Reference recommends the use of the slash to indicate the combination of techniques and the hyphen to indicate the combination of instruments. Thus
- Gas chromatography/mass spectrometry (GC/MS)
- Gas chromatograph-mass spectrometer (GC-MS)
similarly
- time-of-flight mass spectrometry (TOFMS)
- time-of-flight mass spectrometer (TOF-MS)
Ionization methods are set apart by a space, for example
- electron ionization time-of-flight mass spectrometry (EI TOFMS)
Rapid Communications in Mass Spectrometry
The journal Rapid Communications in Mass Spectrometry has in the past given instructions to authors on combined techniques. For example, from the July 12, 2009 RCM:
The Rapid Communications in Mass Spectrometry author guidelines state
- "A single analytical technique, or a type of instrument, is abbreviated without hyphens. Thus, TOFMS, FTICRMS."
- "A hyphen is used when highlighting a particular component or feature of an instrument or technique. Thus, MALDI-TOFMS, ESI-MS/MS. When 2 or more different analytical techniques are coupled in tandem, this is represented by a solidus placed between the abbreviations for the techniques. Thus we write Py/GC/EI-MS, CZE/TOFMS."
Books
- Hyphenated and Alternative Methods of Detection in Chromatography 2012 ISBN 9780849390777
- LC-NMR and Other Hyphenated NMR Techniques 2011 ISBN 9781118135389
- Hyphenated Techniques in Grape and Wine Chemistry 2008 ISBN 9780470061879
- Hyphenated Techniques in Speciation Analysis 2003 ISBN 978-0-85404-545-7
- Data Analysis for Hyphenated Techniques 1996 ISBN 0444822372
- Trac: Directory of Hyphenated Techniques 1994 ISBN 0444821260
- Hyphenated Techniques in Supercritical Fluid Chromatography and Extraction 1992 ISBN 9780444887948
Reviews
- Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects 2013 http://dx.doi.org/10.1002/mas.21377
- Hyphenated separation techniques for complex polymers 2013 http://dx.doi.org/10.1039/C3PY21095B
- Advanced and Hyphenated Techniques for Nano-Level Analysis of Iron in Water 2012 http://dx.doi.org/10.1080/10408347.2012.677720
- Hyphenated liquid chromatography-gas chromatography technique: Recent evolution and applications 2012 http://dx.doi.org/10.1016/j.chroma.2012.02.018
- A critical review on the use of modern sophisticated hyphenated tools in the characterization of impurities and degradation products 2012 http://dx.doi.org/10.1016/j.jpba.2012.03.044
- Multi-Block Polyurethanes via RAFT End-Group Switching and Their Characterization by Advanced Hyphenated Techniques 2012 http://dx.doi.org/10.1021/ma301117k
- Hyphenated techniques as tools for speciation analysis of metal-based pharmaceuticals: developments and applications 2012 http://dx.doi.org/10.1007/s00216-012-5915-9
- Hyphenated chromatographic techniques for structural characterization and determination of masked mycotoxins 2012 http://dx.doi.org/10.1016/j.chroma.2012.02.057
- Overview of hyphenated techniques using an ICP-MS detector with an emphasis on extraction techniques for measurement of metalloids by HPLC-ICPMS 2011 http://dx.doi.org/10.1016/j.microc.2012.03.017
- Introduction to hyphenated techniques and their applications in pharmacy 2010 http://dx.doi.org/10.4103/2229-4708.72222
