Aston 1922/Chapter 6
Chapter VI - Analysis of the Elements
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
50. Arrangement of results
In this Chapter and the one following it are given the experimental results obtained from a large number of elements which have been subjected to analysis with a view to determining their constitution. This Chapter deals with those elements which, by reason of their volatility or properties of forming volatile compounds, can be treated by the ordinary discharge-tube method. The analysis given in all these cases is that obtained by means of the mass-spectrograph.
In Chapter VII will be found the results obtained by the analysis of those elements, all metals, whose positive rays must be generated by special devices. Here the analyses are efifected by several different methods.
The sequence of the elements in the two Chapters is that in which the results were obtained; with the exception of nickel, which is included in the first group although its mass-spectrum was not obtained until after the other metals had been under observation.
51. Oxygen (At. Wt. 16.00) and Carbon (At. Wt. 12.00)
On a mass-spectrum all measurements are relative, and so any known element could be taken as a standard. Oxygen is naturally selected. Its molecule, singly-charged atom, and doubly-charged atom give reference lines at 32, 16, and 8 respectively. The extremely exact integral relation between the atomic weights of oxygen and carbon is itself strong evidence that both are "simple" elements, and so far no evidence appears to have arisen to throw any doubt on this point. Direct comparison of the C line (12) and the CO line (28) with the above standards shows that the whole number relation and additive law hold to the limit of accuracy, i.e. one part in a thousand; and this provides standards C++ (6) C (12), CO (28), and CO2 (44).
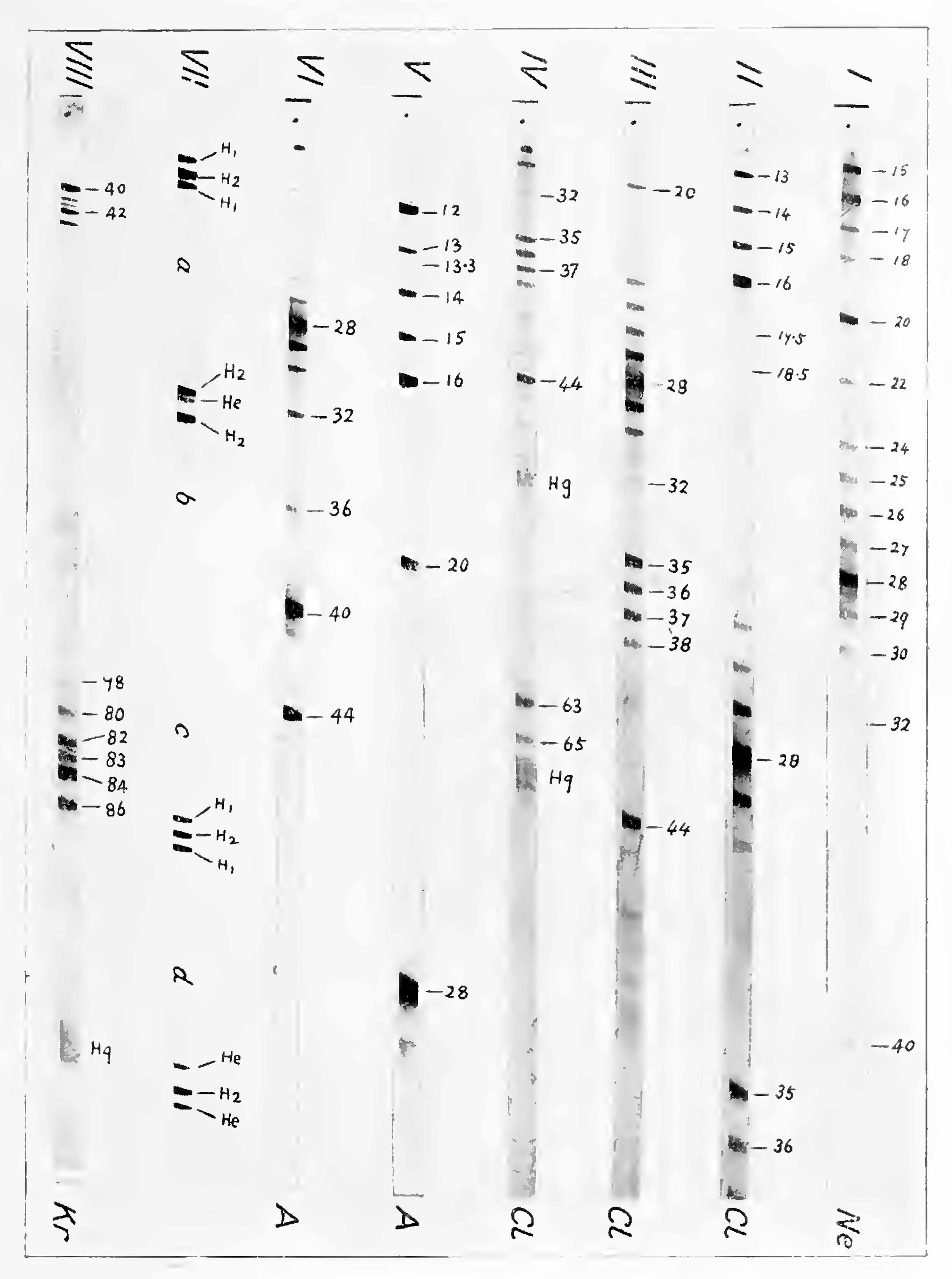
Many of these lines will be recognised on the spectra reproduced on Plate III. The compounds of carbon and hydrogen provide two valuable and easily distinguishable groups of reference lines. The first, which may be called the Ci group, contains five : 12-C, 13-CH, 14-CH2, 15-CH3, 16-CH4 (or O). It is very well shown on Spectrum V, Plate III. When water vapour is present, and particularly when a fresh discharge-tube is used for the first time, it is followed by 17-OH, 18-OH2, and sometimes by 19 presumably OH3 but always very faint. The second hydrocarbon or C2 group contains seven lines : 24, 25, 26, 27, 28, 29, 30, whic h include the very strong and particularly valuable reference line 28 CO or C2H4. This group is well illustrated in Spectra I and II, Plate III. All the above lines may be expected on spectra obtained by the ordinary discharge-tube method; for an addition of CO or CO2 is usually made to the gases or vapours under consideration and assists the smooth running of the discharge. The hydrocarbons are derived from the wax and grease used in the joints of the apparatus.
52. Neon (At. Wt. 20.20)
As soon as the instrument was found to work satisfactorily and enough mass-spectra containing reference lines had been obtained, neon was introduced into the discharge tube. The best results were obtained with a mixture of carbon monoxide and neon, containing about 20 per cent, of the latter gas.
The two first order and two second order lines due to neon were all four available and well placed for measurement on the mass spectra obtained. The following figures are taken from the original paper[1] they are the results of the measurements made on two different plates, using six different spectra.
The method of measuring the position of the lines then in use, combined with a photographic halation effect[2] tended to decrease the masses given by very bright lines. This is enough to account for the reading of the intense 20 Line giving a mass a little too low. The above figures therefore can be accepted as conclusive evidence that neon is a mixture of two isotopes of atomic weights 20.00 and 22.00 (0 = 16) respectively, to an accuracy of about one-tenth per cent.[3]
The two first order lines of neon are shown in Spectrum I, Plate III, but, of course, their relative intensities must not be judged from such a half-tone reproduction. On the original negatives the intensities are in good agreement with the expected ratio 9 to 1 which is necessary to yield the accepted atomic weight 20.20.
53. Possibility of a third isotope of neon
On some of the clearest spectra obtained with neon present there is a distinct indication of a line corresponding to a mass 21. This is an exceedingly faint line and, at first, was thought to indicate the presence of a third isotope. It is now considered more probably due to an abnormal hydride of the kind discussed on page 98.
54. Chlorine (At. Wt. 35-46)
Spectra indicating that this element was a mixture of isotopes were first obtained by the use of hydrochloric acid gas, but as this was objectionable on account of its action on mercury, phosgene (COCl2) was substituted. Spectra II, III, and IV, Plate III, are reproduced from one of the plates taken with this gas. Spectrum I is reproduced for comparison, it shows the state of the tube before chlorine compounds were introduced. It will be seen that chlorine is characterised by the appearance of four very definite lines in the previously unoccupied space to the right of O2 (32): measurement shows these lines to correspond exactly to masses 35, 36, 37, and 38. On Spectrum II, Plate III, taken with a small magnetic field, faint lines will be seen at 17.5 and 18.5. These only appeared when chlorine was introduced, and are certainly second order lines corresponding to 35 and 37. Chlorine is therefore a mixture of isotopes, and two of these have masses 35 and 37. Evidence that Cl35 and Cl37 are the main if not the only constituents is given by the strong lines 63 and 65 (Spectrum IV, Plate III), due to COCl35 and COCl37. The lines 36 and 38 were naturally ascribed to the hydrochloric acids corresponding to Cl35 and Cl37.[4] That this surmise is correct was definitely proved about a year later when the mass spectra of negatively charged rays of chlorine were successfully obtained in the manner described on p. 62, On the negative mass spectra produced in this way only the two chlorine lines 35 and 37 could be distinguished. The property of forming negatively charged ions is a purely chemical characteristic; that isotopes of the same element should differ radically in it is quite out of the question. It is therefore perfectly certain that the lines 36 and 38 are not, to any sensible extent, due to isotopes of chlorine.
On many of the spectra obtained from chlorine compounds a very faint line is distinguishable at 39. This was regarded as a possible third isotope (which would then be an isobare of potassium. No decision on this point has been obtained from the negative mass spectra, for these have, so far, been too faint for the 39 line to be visible, even if it was present. A careful comparison between the intensity of this Hne and those at 35 and 37 on a large number of plates discloses an apparent variation which tells rather decidedly against the idea that a third isotope is present. More evidence, however, will be necessary to clear this point,
55. Argon (At. Wt. 39.88 Ramsay, 39.91 Leduc).
The tube was run with a mixture of CO2 and CH4, and then about 20 per cent, of argon added. The main constituent of the element was at once evident from a very strong line at 40 (Spectrum VI, Plate III), reproduced in the second and third orders at 20 and 13.33 (Spectrum V). The third order line is exceedingly well placed for measurement, and from it the mass of the singly-charged atom is found to be 40.00 ± .02. At first this was thought to be the only constituent, but later a faint companion was seen at 36, which further spectra showed to bear a very definite intensity relation to the 40 line. No evidence drawn from multiple charges was available in this case owing to the probable presence of OH2 and C; but the above intensity relation and the absence of the line from spectra taken just before argon was introduced, made it extremely likely that it was a true isotope.
Any doubt on this point has been removed for all practical purposes by results obtained during the later work on krypton and xenon. Argon was always present to more or less extent during these experiments and the invariable association of a line at 36, of appropriate intensity, with the stronger one at 40 may be regarded as confirming the original conclusion in a satisfactory manner. The presence of 3 per cent, of this lighter isotope is sufficient to reduce the mean atomic weight from 40 to 39.9.
56. Nitrogen (At. Wt. 14.01)
This element shows no abnormal characteristics : its atom cannot be distinguished, on the present apparatus, from CH2 nor its molecule from CO. Its second order line on careful measurement appears to be exactly 7, so it is evidently a simple element, as its chemical combining weight would lead one to expect.
57. Hydrogen (At. Wt. 1.008) and Helium (At. Wt. 3.99)
In connection with the analysis of positive rays the element hydrogen is of peculiar interest in many ways. Its invariable presence in rays generated by the ordinary discharge-tube method, no matter what gas is being employed, is itself a very striking phenomenon, even when due allowance has been made for its abnormal power in affecting screens and plates.
The ease with which its brilliant lines, the molecular one in particular, can be generated and observed visually is of an importance hardly to be exaggerated in the development and technique of the mass-spectrograph. The advantage of the visible presence of the H2 line has already been referred to[5] and was realised very keenly in the investigation of the alkali metals when the method precluded the use of this line to indicate when suitable conditions for exposure had been obtained.[6]
The hydrogen atom is the lightest particle ever observed to carry a positive charge, which agrees very well with the generally accepted idea that the true Moseley number of this element is 1. This implies that the neutral atom of hydrogen only contains one electron and therefore can only acquire a single positive charge in losing it. The singly charged particle so formed is therefore the "proton" or ultimate atom of positive electricity itself.
Helium, on the other hand, can lose two electrons and acquire a double charge, indeed its atoms are invariably in this state when ejected from the nuclei of radioactive elements as alpha rays. Nevertheless, in spite of every effort to obtain the second order Une of helium for direct comparison with the hydrogen molecule not the faintest indication of it h as yet been observed on a mass spectrum, although there is not the least difficulty in obtaining its first order line to any intensity required.
The explanation of this is probably to be found in the very high ionisation potential about 80 volts[7] — associated with the detachment of both electrons. If doubly charged helium atoms are formed in the discharge tube — and we have every reason to consider this probable — their chance of passing through the slit system and the deflecting fields without picking up a single electron may be practically nil. This is made the more likely by the fact that helium is not absorbed by charcoal and liquid air, so that when it is present the pressure in the apparatus tends to become undesirably high.
58. The determination of the masses of atoms of hydrogen and helium by the method of "Bracketing"[8]
The determination of masses so far removed as these from the ordinary reference lines offers peculiar difficulties, but, as the lines were expected to approximate to the terms of the geometrical progression 1, 2, 4, 8, etc., the higher terms of which are known, a special method was adopted by which a two to one relation could be tested with some exactness. Two sets of accumulators were selected, each giving very nearly the same potential of about 250 volts. The potentials were then made exactly equal by means of a subsidiary cell and a current-divider, the equality being tested to well within 1 in 1000 by means of a null instrument. If exposures are made with such potentials applied to the electric plates first in parallel and then in series, the magnetic field being kept constant, all masses having an exact two to one relation will be brought into coincidence on the plate.[9] Such coincidences cannot be detected on the same spectrum photographically; but if we first add and then subtract a small potential from one of the large potentials, two lines will be obtained which closely bracket the third. To take an actual instance using a gas containing hydrogen and helium, with a constant current in the magnet of 0.2 ampere, three exposures were made with electric fields of 250, 500 + 12, and 500 - 12 vol respectively. The hydrogen molecule line was found symmetrically bracketed by a pair of atomic lines (Plate III, Spectrum VII, a and c), showing within experimental error that the mass of the molecule is exactly double the mass of the atom. When after a suitable increase of the magnetic field the same procedure was applied to the helium line and that of the hydrogen molecule, the bracket was no longer symmetrical (Spectrum VII, 6), nor was it when the hydrogen molecule was bracketed by two helium lines (d). Both results show in an unmistakable manner that the mass of He is less than twice that of H2. In the same way He was compared with 0++ and H2.[10] The method is discussed on p. 57. The values obtained by its use can be checked in the ordinary way by comparing He with C++ and H3 with He, these pairs being close enough together for the purpose. The following table gives the range of values obtained from the most reliable plates:
From these figures it is safe to conclude that hydrogen is a simple element and that its atomic weight, determined with such consistency and accuracy by chemical methods, is the true mass of its atom.
This result leads to theoretical consideration of the greatest importance, which will be discussed later.[11]
59. Triatomic Hydrogen H3
The occurrence of a parabola corresponding to a mass 3 was first observed and investigated by Sir J. J, Thomson.[12] He came to the conclusion that it was probably due to triatomic hydrogen. The simplest way of obtaining this substance is to bombard KOH with cathode rays and pump off the gases so produced. The H3 used for the above measurements was obtained in this way. The mass deduced proves in a conclusive manner that the particle causing it is a molecule of three hydrogen atoms, a result independently established about the same time by the chemical work of Wendt and Landauer.[13]
60. Krypton (At. Wt. 82.92) and Xenon (At. Wt. 130.2)
The results with these elements were particularly interesting. The first source available, was the remains of two small samples of gas from evaporated liquid air. Both contained nitrogen, oxygen, argon, and krypton, but xenon was only detected spectroscopically in one and its percentage in that must have been quite minute. Krypton is characterised by a remarkable group of five strong lines at 80, 82, 83, 84, 86, and a faint sixth at 78. This cluster of isotopes is beautifully reproduced with the same relative values of intensity in the second, and fainter still in the third order. These multiply-charged clusters give most reliable values of mass, as the second order can be compared with A (40) and the third with CO or N2 (28) with the highest accuracy. It will be noted that one member of each group is obliterated by the reference line, but not the same one. The singly and doubly charged krypton clusters can be seen to the right and left of Spectrum VIII, Plate III. It will be noticed that krypton is the first element examined which shows unmistakable isotopes differing by one unit only.
On the krypton plates taken with the greatest magnetic field faint, but unmistakable indications of fines in the region of 130 could just be detected. The richest sample was therefore fractionated over liquid air, and the last fraction, a few cubic millimetres, was just sufficient to produce the xenon liines in an unmistakable manner. Five could be distinguished, but owing to difficulties in the way of accurate measurement the provisional values first published were one unit too low.
Later on in March, 1921, a sample of gas was obtained which contained a large proportion of xenon, though it was by no means free from krypton. This yielded some excellent mass spectra, which not only served to correct the figures given for the five isotopes discovered previously, but also indicated the possibility of two additional ones.
The absolute position of the group on the mass scale was satisfactorily fixed by means of the second order line of the strongest member, which fortunately lies outside the third order mercury group. This gave constant and accurate values corresponding to 64.5. The five strong fines of xenon are therefore 129, 131, 132, 134, 136. On the left of the first there was to be seen on many of the plates distinct indications of a faint component 128. Also the darkening between the lines 129 and 131 appears decidedly greater than that to be expected from ordinary halation and suggests the possibility of a seventh isotope 130. The relative intensity of the lines of krypton and xenon is best indicated in Fig 17, p. 109.
61. Mercury (At. Wt. 200.6)
As this element is employed both in the apparatus for the admission of gas and in the Gaede vacuum pump, it would be very difficult to eliminate it entirely from the discharge. This is fortunately neither necessary nor desirable in most cases, for it provides a valuable reference scale and, for some reason unknown, its presence is definitely beneficial to the smooth running of the discharge tube.
Mercury is abnormal in its capacity for forming multiply charged particles. A study of its remarkable parabolas enabled Sir J. J. Thompson to show that the atom of mercury can carry no less than eight charges, that is lose eight electrons. He gives reasons for considering that it loses all eight at once and then recaptures them one at a time, so giving rise to a series of parabolas 200/1, 200/2, 200/3, etc. The brightest is the first, which is due to atoms which have recaptured all but one electron; the others are progressively fainter.
Subjected to the greater resolving power of the mass spectrograph it was seen at once that mercury was a complex element. Its first, second, third, and higher order fines appeared as a series of characteristic groups around positions corresponding to masses 200, 100, 66⅔, etc. Some of these will be easily distinguished on the spectra reproduced. The second, third and fourth order groups are well shown in Spectrum VIII, Plate IV. Careful study of the group shows that it consists of a strong line 202, a weak one 204 and a strong group 197-200 which cannot be resolved on the present instrument, but which in all probability contains all the four integers in that range.
62. Boron (At. W. 10.90) Fluorine (At. W. 19.00). Silicon (At. W. 28.3).
It will be convenient to treat of these three elements together. The atomic weights of boron and fluorine have both been recently redetermined by Smith and Van Haagen[14] with the above results. On the atomic weight of silicon there is some divergence of opinion. The international value is quoted above, but Baxter, Weatherell, and Holmes make it nearer 28.1.[15]

After a failure to obtain the boron lines with some very impure boron hydride, a sample of boron trifluoride was prepared from boric acid and potassium borofluoride, and this gave good results. Following the usual practice, it was mixed with a considerable quantity of CO 3 before introduction into the discharge-tube. Very complex and interesting spectra were at once obtained, and it was remarked that this gas possessed an extraordinary power of resurrecting the spectra of gases previously used in the apparatus. Thus the characteristic, first and second order lines of krypton were plainly visible, although the tube had been washed out and run many times since that gas had been used. This property of hberating gases which have been driven into the surface of the discharge bulb is doubtless due to the chemical action of the fluorine, hberated during the discharge, on the siUca anticathode and the glass walls. After running some time the corrosion of the anticathode was indeed quite visible as a white frost over the hottest part.
After several successful series of spectra had been secured, the percentage of boron trifluoride in the gas admitted was increased as far as possible, until the discharge became quite unmanageable and the tube ceased to work. Just before it did, however, it yielded two very valuable spectra which confirmed the isotopic nature of boron. These are reproduced side by side as they were taken (Spectra I & II) Plate IV. The fines at 10 and 11 are undoubtedly both first-order lines of boron. The hypothesis that these might be due to neon liberated by the action mentioned is not tenable, both on account of their relative intensities and the absence of strong neon first-order lines. Even if it were, it could not explain the presence of the well-defined lines at 5 and 5.5 which had never been obtained before at all, and which must be second-order lines of boron. This element therefore has at least two isotopes 10 and 11. The relative photographic intensity of the lines 5 and 5.5 does not agree well with an atomic weight as high as 10.9, and the discrepancy might be explained by the presence of a third isotope at 12; which would be masked by carbon, for it has not yet been found practicable to eliminate carbon from the discharge. But Plate IV, Spectrum IV, contradicts this suggestion for, as will be shown later, the line at 49 is mainly if not wholly due to B11F2, so that there should also be a line at 50 for B12F2. The line at 49 is very strong, but at 50 any small effect there may be can safely be ascribed to the fourth order of mercury. The evidence is clearly against the presence of a third isotope of boron.
The exceedingly accurate whole-number value for the atomic weight of fluorine suggests the probability of this element being simple. This conclusion is borne out by the strong line at 19.00 with second-order line at 9.50. The accompanying line at 20, very faint in Spectrum II, Plate IV, is no doubt HF. As there is no evidence whatever to the contrary, fluorine is taken to be a simple element with an atomic weight 19.
Having adopted these values for boron and fluorine, we may now apply them to Spectra III and IV, Plate IV, taken with boron trifluoride. Consider first the group of three very strong lines 47, 48, and 49. The last two are to be expected as being due to B10F2 and B11F2 respectively, but since there is no evidence of a boron 9 or a fluorine 18, line 47 cannot be due to a compound of these elements. But line 47 only appeared when BF3 was introduced, and so must be due to silicon fluoride formed by the action of the fluorine on the glass walls and the silica anticathode.
To test this the BF3 was washed out and replaced by SiF,, which had been made by the action of sulphuric acid on calcium fluoride and silica in the usual way. This greatly reduced the lines 48 and 49, and so they must be attributed to boron compounds. At the same time line 47 remained very strong, and was evidently due to a compound Si28F, so that silicon has a predominant constituent 28. This conclusion is further supported by the presence of very strong lines at 66, Si28F2 and 85, Si28F3.
The chemical atomic weight shows that this cannot be its only constituent. Lines at 29, 48, 67, and 86 all suggest a silicon of atomic weight 29. Practically conclusive proof of this is given in Spectrum V, Plate IV, which shows its second order line unmistakably at 14.50. The only other reasonable origin of this line, namely second-order B10F, is eliminated by the fact that there is no trace of a line at 10 in this spectrum. The evidence of a silicon of atomic weight 30 is of a much more doubtful character. Its presence is suggested by the lines 30, 49, 68, and 87, but the possibility of hydrogen compounds makes this evidence somewhat untrustworthy, and no proof can be drawn from a second-order line 15, as this is normally present and is due to CH3. On the other hand, if we accept a mean atomic weight as high as 28.3, the relative intensity of the lines due to compounds of Si28 and Si29 indicates the probable presence of an isotope of higher mass. These considerations taken with the complete absence of any definite evidence to the contrary make the possibility of Si30 worth taking into account.
63. Molecular lines of the Second Order
The work of Sir J. J. Thomson on multiply-charged positive rays showed very definitely that molecules carrying more than one charge were at least exceedingly rare,[16] for not a single case was observed which could not be explained on other grounds. Up to the time of the experiments with the fluorine compounds the same could be said of the results with the mass-spectrograph. This absence of multiply-charged molecular fines, though there is no particular theoretical reason for it, has been used as confirmatory evidence on the elementary nature of doubtful liines.
The spectra obtained with BF3 show lines for which there appears no possibility of explanation except that of doubly charged compound molecules. The two most obvious of these may be seen on Plate IV, Spectrum III, and at the extreme left-hand end of Spectrum IV. They correspond to masses 23.50 and 24.50, the first being quite a strong fine. Were there no fines of lower order corresponding to these, the whole-number rule might be in question; but all doubt is removed by the fact that the lines 47 and 49 are two of the strongest on the plate. A comparison of several spectra upon which these lines occur shows a definite intensity relation which practically confirms the conclusion that the first pair of lines are true second-order lines corresponding to the first order lines of the second pair. Now fines 47 and 49 cannot by any reasonable argument be elementary, they must in fact be due to compounds of fluorine with boron B11F2 or silicon Si28F, or due to both. Further evidence of the capability of fluorine compounds to carry two charges is offered by fine 33.50, which is undoubtedly the second-order fine corresponding to 67, i.e. B10F3 or Si29F2. So far as results go, fluorine appears to be unique in its power of yielding doubly-charged molecules in sufficient number to produce second-order liines of considerable strength.
64. Bromine (At. Wt. 79.92).
The results with this element were definite and easy to interpret. Its chemical combining weight is known with great certainty, and is very nearly the whole number 80. It was rather a surprise, therefore, that it should give a mass-spectrum which showed it to consist of a mixture of two isotopes in practically equal proportions. Methyl bromide was used for the experiments, and one of the results is reproduced in Plate IV, Spectrum VI. The characteristic group consists of four fines at 79, 80, 81, and 82. 79 and 81, apparently of equal intensity, are much the stronger pair, and are obviously due to elementary bromines. This result is practically confirmed by second-order fines at 39.5 and 40.5 too faint to reproduce, but easily seen and measured on the original negative. The fainter pair, 80 and 82, are the expected lines of the two corresponding hydrobromic acids.
65. Sulphur (At. Wt. 32.06)
Spectra VII and VIII, Plate IV, show the effect of the addition of sulphur dioxide to the gas in the discharge-tube. Above each is a comparison spectrum taken immediately before the gas was admitted, on the same plate with approximately the same fields. The very marked strengthening of fines 32 and 44 is no doubt due to S and CS. New fines appear at 33 SH, 34 SH2, 60 COS, 64 SO2 or S2, and 76 CS2. It may be noticed that lines 32, 60 and 76 are accompanied by a faint line one unit higher and a rather stronger line two units higher. In the first case it is certain and in the others probable that these are, at least partly, due to hydrogen addition compounds. If a higher isotope of sulphur exists, as is suggested by the chemical atomic weight, it seems unlikely that this should have mass 33, for this would have to be present to the amount of 6 per cent., and should give a line at 35 one-thirteenth the strength of 34 (normal SH2). No such line is visible. A sulphur of atomic weight 34 present to the extent of 3 per cent, is more likely, but there is hardly enough evidence as yet to warrant its serious consideration.
66. Phosphorus (At. Wt. 31.04), Arsenic (At. Wt. 74.96).
The gases phosphine PH3 and arsine AsH3 were used in the experiments on these elements, and the results were of notable similarity. The mass-spectrum of each gas was characterised by a group of four lines. The first and strongest doubtless due to the element itself, the second rather weaker due to the monohydride, the third very faint to the dihydride, and the fourth fairly strong to the trihydride. The spectrum of AsH3 is shown in Spectrum IX, Plate IV; that of phosphorus is similar but its lines are weak, and therefore unsuited to reproduction. Both elements appear to have no isotopes, and neither give visible second-order lines.
67. Selenium (At. Wt. 79.2), Tellurium (At. Wt. 127.5).
The compounds used in the experiments on these elements were selenium hydride, made by passing a stream of hydrogen through boiling selenium, and tellurium methyl. Complete failure resulted in both cases. There was, indeed, on one spectrum an exceedingly faint line at 79, but no shred of reliable evidence could be found to ascribe it to an isotope of Se. In the case of tellurium no trace of any fine near 127 could be discovered. The failure is unfortunate in the case of Te on account of its well-known anomalous position in the periodic table; in the case of Se particularly so for the following reasons: If the accepted atomic weight is even approximately correct this element must have one isotope, at least, of atomic weight greater than 78. But the numbers 79, 80, 81, 82, 83, 84, are already filled by isotopes of Br and Kr, so that it is extremely probable that one of the isotopes of Se has an atomic weight identical with one of an element having a different atomic number, i.e. is an Isobare. The latter are known to exist among radioactive elements, but none have so far been discovered during the work on mass spectra.
68. Iodine (At. Wt. 126.92)
The results with this element were fortunately both definite and conclusive. Methyl iodide was employed, its vapour being introduced mixed with CO3 and CH3. It gave one strong line at 127 satisfactorily confirmed by another single line at 142 due to CH3I.
This proves iodine to be a simple element in an unequivocal manner, a rather unexpected result since all the speculative theories of element evolution, by Van den Broek and others, predict a complex iodine.
69. Antimony (At. Wt. 120.2)
Antimony hydride SbH3 was used. This was made by dissolving antimony magnesium aUoy in dilute acid. Unlike the corresponding arsenic compound it gave an entirely negative result, no fine whatever being distinguishable in the region expected from the atomic weight. This failure is probably to be ascribed to the exceedingly unstable nature of the antimony compound.
70. Tin (At. Wt. 118.7)
Tin tetrachloride was employed in the investigation of this element. The vapour of this compound attacks the tap grease used in the apparatus, which makes it extremely difficult to deal with. The results were entirely negative except in one case. On this occasion a second attempt to get the selenium fine from selenium hydride was actually in progress, but a good deal of SnCl4 vapour had been introduced previously, and the chlorine lines were so intense that some "resurrected" compound of chlorine must have been the principal factor in the discharge. For some unknown reason the discharge tube was working abnormally well. On one of the spectra then obtained. Spectrum II, a group of lines of even integral mass 116, 118, 120, 122, 124 (followed by iodine 127) could be distinguished and some of these may possibly have been due to isotopes of tin. This supposition is slightly strengthened by the appearance of a still fainter group of odd integral mass containing the lines 155, 157, etc., which might be isotopic tin monochlorides. It has not been found possible to repeat this result, so that no reliance is to be put upon it.
71. Nickel (At. Wt. 58.68)
Nickel received attention early in the history of positive rays as it is one of the elements whose atomic weight is out of order in the periodic table; it should be heavier, not lighter than cobalt (58.97). It is amenable to treatment in the ordinary discharge tube for it forms an easily vaporisable carbonyl compound Ni(C0)4. Unfortunately this is very rapidly decomposed by the electric discharge, so that in the early experiments made by Sir J. J. Thomson the walls of the discharge bulb became coated with a black deposit of the metal, it was impossible to maintain a steady discharge for a sufficient time, and no satisfactory parabola corresponding to the element could be obtained.
Quite recently[17] by the use of abnormally high current intensities in the discharge it has been found possible to overcome these difficulties to some extent and to obtain a satisfactory mass spectrum from a mixture of nickel carbonyl and carbon dioxide. This consists of two lines, the stronger at 58 and the weaker at 60. They are most conveniently placed between the mercury groups of the third and fourth order, with which they can be compared with an accuracy of one-tenth per cent. The results were also checked by comparison with the CO2 line at 44, and appear to be integral within the above error. Nickel therefore consists of at least two isotopes. The intensities of the lines are about in the ratio 2:1, and this agrees with the accepted atomic weight. It may be noticed that had the heavier isotope preponderated the atomic weight of the element would have appeared normally placed in the periodic table.
References
- ↑ 1 Aston, Phil. Mag., 39, 454, 1920.[1]
- ↑ V. p. 60.
- ↑ Aston, Nature, Nov. 27, 1919; Phil. Mag. 39, 464, 1920
- ↑ Aston, Nature, Dec. 18, 1919; Phil. Mag., 39, 611, 1920.
- ↑ V. p. 52.
- ↑ V. p. 87.
- ↑ Franck and Knipping, Phys. Zeit., 20, 481, 1919; Ver. Deut. Phys. Qes. 20, 181, 1919; and Horton and Da vies, Proc. Roy. Soc. 95A, 408, 1919; Phil. Mag. 39, 692, 1920.
- ↑ 1 Aston, Phil Mag. 39, 621, 1920.
- ↑ V. p. 57.
- ↑ V. p. 70.
- ↑ V. p. 100.
- ↑ J. J, Thomson, Rays of Positive Electricity, p. 116, 1913.
- ↑ Wendt and Landauer, Jour. Am. Chem. Soc, 42, 930, 1920.[2]
- ↑ Smith and Van Haagen, Carnegie Inst. Washington Publ. No. 267, 1918.[3]
- ↑ Baxter, Weatherell and Holmes, Jour. Am, Chem. Soc, 42, 1194, 1920.[4]
- ↑ J. J. Thomson, Rays of Positive Electricity, p. 64.
- ↑ Nature June 23, 1921, p. 520.
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
