Aston 1922/Chapter 7
Chapter VII - Analysis of the Elements (Continued)
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
72. Positive Rays of Metallic Elements
Positive rays of most of the metallic elements cannot be obtained by the ordinary discharge-tube method, since in general they have extremely low vapom'-pressures and are incapable of forming stable volatile compounds. Mercury is a notable exception to this rule, and its rays are exceedingly easy to produce.
Positively charged rays which appeared to be atoms of the alkali metals were first observed by Gehrcke and Reichenheim.[1] They obtained them by two distinct methods: the first, which may be conveniently called the "Hot Anode" method, consisted in using as anode of the discharge-tube a platinum strip coated with a salt of the metal and electrically heated by an external battery. The second device, with which they performed most of their pioneer work on Anode Rays, was to use a composite anode of special construction which worked without the need of external heating.
73. Dempster's analysis of Magnesium (At. Wt. 24.32)
The experiments of Dempster with the "hot anode" method of generating positive rays have already been noted.[2] Later,[3] he announced the very important discovery of the three isotopes of magnesium, and subsequently published an account of the experimental details,[4] The magnesium rays were obtained from a piece of the metal which was heated electrically by a coil of wire, and at the same time bombarded by electrons from a Wehnelt cathode. The occluded gases were first driven off, and then the heating current was increased till the metal was slightly vaporised and the magnesium lines appeared. The following description of the analysis and the curves obtained are taken direct from Dempster's paper:
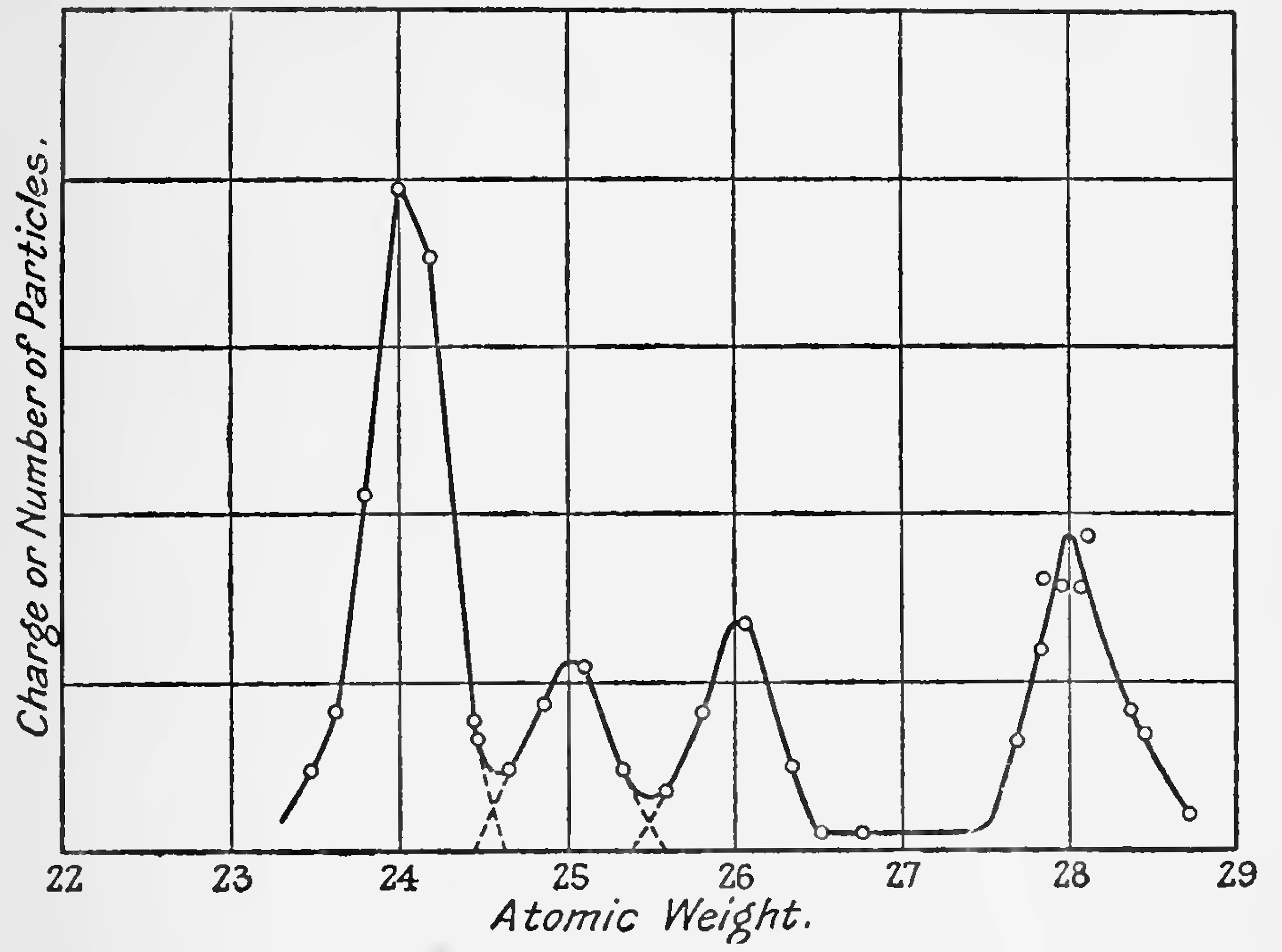
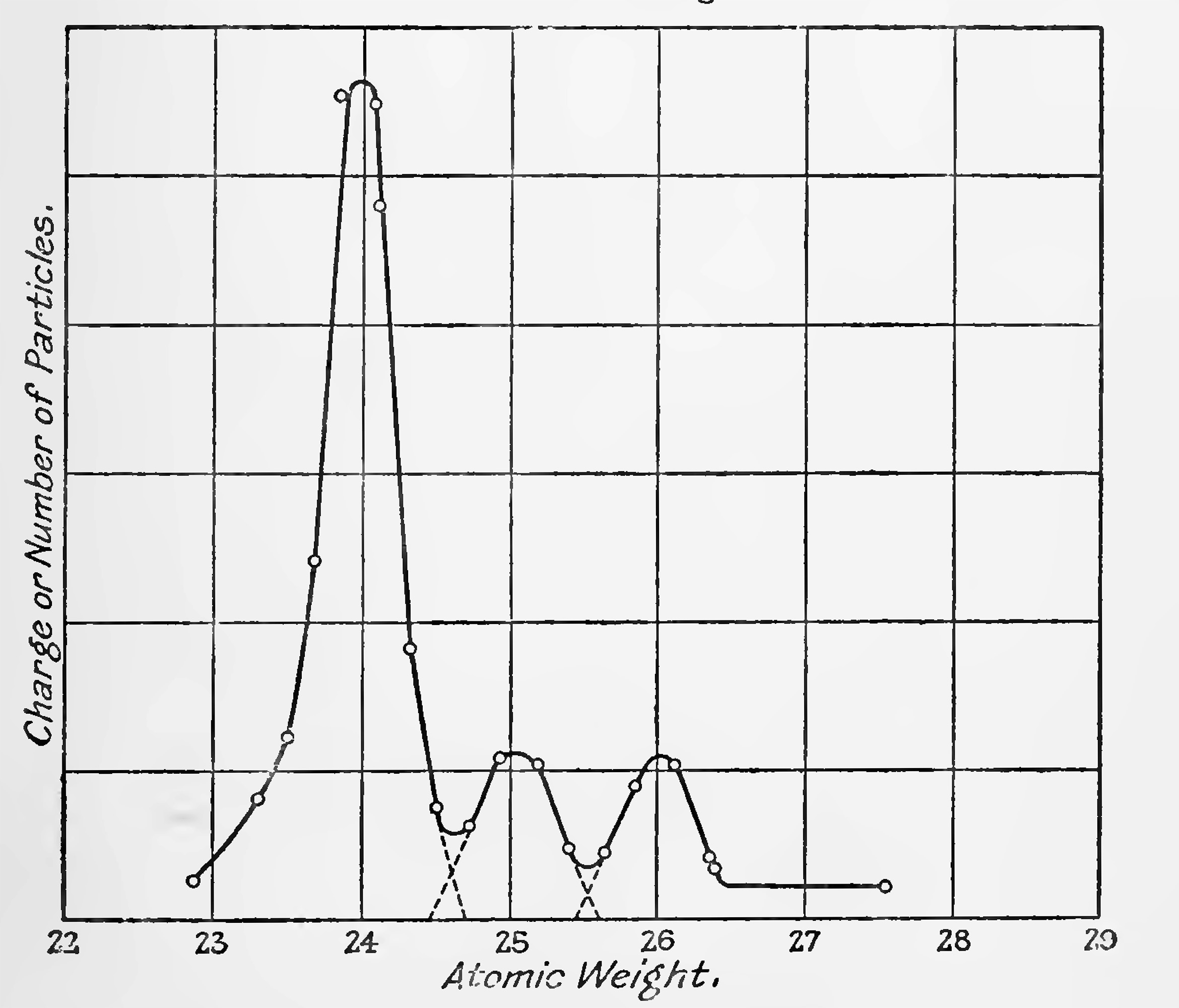
The charged atoms of different atomic weights are successively brought on to the detecting electrode by keeping the magnetic field constant and varying the potential which accelerates the rays, the potential required being inversely proportional to the mass of the particles. Thus, if one atomic weight is known the others may be found. Due to the finite width of the slits, each element gives a curve, on the atomic weight scale, which is theoretically a linear increase to a maximum and then a Linear decrease. The width half way to the maximum is given by m. where m is the atomic weight, S the slit width and d the diameter of the circle in which the rays travel. Under good vacuum conditions this theoretical sharpness is practically obtained. For 1 mm. slits this width of the curves should thus be one-half a unit on the atomic weight scale. The former measurement with the apparatus and the magnetic field determinations sufficed to locate elements between 20 and 30 within one unit, and identified the strong nitrogen rays (possible carbon monoxide) of molecular weight 28 which are given off when the metal is first heated.
"One series of experiments was as follows. After heating the magnesium slightly and pumping, till a MacLeod gauge gave no pressure indication, the nitrogen molecule was the only particle present. The heating current was then increased by steps to vaporise the magnesium. With 0.7 ampere, 28 alone was present, with 075 ampere an arc apparently struck as the cathode-anode current jumped suddenly to five times its value. The electron current was decreased to its former value by cooling the cathode and the rays were measured. It was found that three strong new lines had appeared. The new lines which are undoubtedly due to magnesium were compared with the nitrogen rays which were still faintly present and found to have atomic weights, 24, 25 and 26. The observations are illustrated in Fig. 13, which gives the current or number of particles for different atomic weights. The nitrogen line had its maximum at 817 volts, and the atomic weight abscissae are 28 x 817 divided by the volts applied. The ordinates of the 28 line are multiplied by 10 in plotting to make them comparable with the other three lines. The dotted continuation to the axis indicates the slight overlapping of the Lines. We conclude that magnesium consists of three isotopes of atomic weights 24, 25 and 26.
"Later curves made with steadier discharge conditions are more suitable than Fig. 13 for measuring the relative strengths of the components. In Fig. 13 there appears to have been a drop in intensity just before 24 was reached, in the measurement from high to low atomic weights. The curve is of interest as still containing 28 faintly and so serving accurately to locate the weights which otherwise would have been uncertain to a fraction of a unit.
"Fig. 14 is one of several later curves taken under steadier conditions. These all have very closely the same appearance. The components 25 and 26 are present very nearly in equal amounts; in some measurements 25 was found about nine tenths the intensity of 26. The component at 24 is approximately 6 times as strong as the one at 26. The ratio of 1 : 1 : 6 gives an average atomic weight 24.375, which is in as good agreement with the accepted atomic weight for magnesium as could be expected with the wide slits used in these first experiments."
74. The mass-spectra of the alkali metals
In order to analyse the metals of this group a modification of Gehrcke and Reichenheim's hot anode method was employed by the writer to generate the positive rays. After a certain amount of initial difficulty in technique had been overcome this gave satisfactory results.[5]
The apparatus for producing the rays was very simple, and will be readily understood from the figure (Fig. 15). The hot anode A is a strip of platinum foil .03 mm. thick, about 2 mm. wide by 7 mm. long, welded to the two stout platinum leads which are fused through the glass at C. It was raised to the required temperature by current from one large storage-cell connected through a rheostat as shown. As the anode is of necessity the high-potential pole of a discharge-tube arranged to give positive rays, this heating arrangement had to be very carefully insulated. The anode was mounted on a ground joint as indicated so that it could be easily removed and replaced. The discharge-tube was cylindrical, about 4 cm. in diameter, mounted concentric to the axis of the perforated cathode K. A side tube was fitted at B which could be cooled in liquid air; in some of the experiments this was charged with charcoal.

The anode was placed immediately opposite the perforation of the cathode and about 1 cm. away from it. The platinum strip was bent at one end into a U-shaped channel into which the salts could be melted. The discharge was maintained by a large induction-coil used in the previous work on mass spectra and rectified by means of a valve V.
75. Experiments with the Parabola method of analysis
In the preliminary experiments the analysis of the rays was performed by Sir J. J. Thomson's "parabola" method, since this gives the maximum general information, and it was only when suitable conditions and technique had been ascertained that the mass spectrograph was applied.
The general procedure was to pump out the discharge-tube to the lowest possible pressure, far lower than that necessary to prevent all discharge with the anode cold, and then to heat up the anode until the discharge started. This usually happened at dull red heat, and by very careful adjustment of the temperature and of the primary current in the coil it was possible, under favourable conditions, to maintain a fairly steady current of 1 to 2 milliamperes at a potential of about 20,000 volts.
It will be seen that the arrangement resembles that of a Coolidge X-ray tube reversed pole for pole, and it was hoped that it might share the outstanding controllability of that device; but that expectation was only very partially realised,
The mechanism of the discharge is extremely obscure, for the current intensity is, of course, enormously in excess of that to be expected from the ordinary thermionic release of positive ions from the hot anode.[6] There was very little visible glow in the tube, the X-radiation was small and, although a faint cloud of sodium light nearly always appeared in front of the red-hot anode, the pressure was too low for the anode rays to be visible; their point of impact with the cathode could, however, be inferred from the scintillations on its surface.
Observations of this effect lead to the conjecture that the bulk of the rays originate not from the surface of the salt itself but from that of the heated platinum, and also that some points on this are much more active than others, giving rise to jets of rays. The direction of these jets seemed to depend on the local configuration of the strip and was beyond practical control. The obvious device of moving the anode about by means of the ground joint to get a radiant point in the required place could not be applied, for the parabolas were never bright enough to be visible on the willemite screen. To add to these difficulties the salt disappeared very rapidly, in some cases in a few minutes. Consequently exposures were very limited in duration, and even in the most favourable cases the results rarely had a satisfactory intensity.
The preliminary experiments were done with sodium phosphate, and before long encouraging results were obtained. In all the successful exposures only a single parabola appeared, and this showed that although the method on account of the number of inevitable failures is an exasperating one to use as a means of identifying isotopes it has the great merit of producing the positive rays of the metals and no others. This characteristic seems to be due to the very low pressure employed and also possibly to the position of the anode itself, which prevents any positive rays generated in more distant parts of the tube from ever reaching the perforation in the cathode in the necessary axial direction.
Such a selective action has two very important results. In the first place, it eliminates the many ambiguities of the ordinary mass spectrum due to multiply-charged rays, or to hydrogen and other addition products; but, in the second, it prevents the use of the oxygen line as a comparison standard. As soon as it was demonstrated beyond any reasonable doubt that sodium was a simple element (and its chemical atomic weight is so exactly integral on the oxygen scale as to be conclusive corroboration) it was taken as standard at 23.
76. Lithium (At. Wt. 6.94)
The most successful experiment done with the parabola method of analysis was one in which a mixture of sodium and lithium phosphates was employed (this contained traces of potassium salts). By great good fortune a very strong jet of rays must have been directed along the axis and three satisfactory exposures were obtained before the anode dried up. One of these is reproduced in Plate I (5) A strong parabola at 7 and a weak one at 6 demonstrate clearly that lithium is a complex element, as its chemical atomic weight 6.94 leads one to expect. This result, which was announced by the writer and G. P. Thomson in Nature, February 24th, was confirmed independently by Dempster[7] using the method described for magnesium. The several photographs here considered all gave approximately the same ratio of intensities, and they corresponded as well as was to be expected with the accepted atomic weight. On the other hand, G. P. Thomson's parabolas (which were obtained with a composite anode) and Dempster's electrical measurements suggest a more nearly equal intensity ratio and this ratio appears to vary.
77. Sodium (At. Wt. 23.00)
Sodium gave the brightest effects, and its single line was obtained so intense that the presence of another constituent to the extent of even less than 1 per cent, could probably have been detected. It may therefore be safely regarded as a simple element.
The parabola method of analysis is perfectly satisfactory in the case of so light an element as lithium, but cannot be used for the critical examination of the heavier members of the group; and so the apparatus for the production of the rays was fitted, to the mass spectrograph already described.[8] The experimental difficulties became now very serious indeed, for, in addition to those already indicated, there was no means of finding the most suitable voltage to apply to the electrostatic plates. In normal cases this is done by visual inspection of the hydrogen lines, but here it could only be guessed at. Under these conditions it is not a matter for surprise that the photographs, though sufficient for the purpose of detecting isotopes, only gave very faint lines and so cannot be reproduced as illustrations.
78. Potassium (At. Wt. 39.10)
A mixture of potassium sulphate, potassium bromide, and a little sodium phosphate was now used on the anode, and after several unsuccessful attempts some fairly satisfactory spectra were obtained which contained both sodiun and potassium lines. Using the former as standard the latter consisted of a bright component at 39, and a very faint component at 41.
79. Rubidium (At. Wt. 85.45)
Rubidium chloride was now added to a little of the mixture used in the potassium experiments and spectra containing the potassium and rubidium lines were obtained. Rubidium is very definitely double. Its components are more nearly equal in intensity than those of lithium or potassium. Measured against the potassium line 39 its stronger component is 85 and the weaker 87. The intensity ratio agrees reasonably well with the accepted atomic weight 85.45.
80. Caesium (At. Wt. 132.81)
Then a mixture of rubidium chloride and caesium chloride was used evidence of a line at 133, measured against the two rubidium lines, was soon obtained. Pure caesium chloride was then substituted and the utmost possible exposure given to search for a lighter component, which was to be expected from the fractional chemical atomic weight 132.81, Although by this means the intensity of the line 133 was increased to a satisfactory pitch no other neighbouring line was found. If, therefore, a lighter isotope of caesium exists it must differ from 133 by many units which seems very unlikely or it cannot be present in proportion sufficient to account for the fractional atomic weight obtained by chemical means.
81. Thompson's work on Beryllium (At. Wt. 91)
G. P. Thomson[9] has recently investigated the Anode rays obtained from a composite anode similar to that devised by Gehrcke and Reichenheim[10] and has subjected them to analysis by the parabola method. After the parabolas of the isotopes of lithium had been successfully obtained[11] he went on to investigate the element beryllium. The best results were obtained from a mixture of sodium bromide and berylium fluoride. This gave a single strong parabola corresponding to an atomic weight 9 (Na = 23). The accepted chemical atomic weight is rather higher, so a careful examination was made to discern any possible faint companions at 10 or 11. He concludes that neither of these can be present to any sensible extent, and therefore that beryliium is probably a simple element.
82. Calcium (At. Wt. 40.07) and Strontium (At. Wt. 87.63)
Thomson also obtained by the same method parabolas due to these elements, the latter very faint, but the resolution at his disposal was too low to decide their constitution. From the position of the strong parabola of calcium he concludes that one or more of the atomic weights 39, 40, 41 were present; and as all these are already known to exist as isotopes of either potassium or argon, it follows that calcium must be an isobare of one or other of these elements.[12]
83. Table of Elements and Isotopes
The following list tabulates the results contained in this and the previous Chapter.
The isotopes of complex elements are given in the order of the proportions present. Brackets indicate that the figures are provisional only.
Table of Elements and Isotopes
| Element. | Atomic number. | Atomic weight. | Minimum number of isotopes. | Masses of isotopes in order of intensity. |
|---|---|---|---|---|
| H | 1 | 1.008 | 1 | 1.008 |
| He | 2 | 4.00 | 1 | 4 |
| Li | 3 | 6.94 | 2 | 7, 6 |
| Be | 4 | 9.1 | 1 | 9 |
| B | 5 | 10.9 | 2 | 11, 10 |
| C | 6 | 12.00 | 1 | 12 |
| N | 7 | 14.01 | 1 | 14 |
| O | 8 | 16.00 | 1 | 16 |
| F | 9 | 19.00 | 1 | 19 |
| Ne | 10 | 20.20 | 2 | 20, 22, (21) |
| Na | 11 | 23.00 | 1 | 23 |
| Mg | 12 | 24.32 | 3 | 24, 25, 26 |
| Si | 14 | 28.3 | 2 | 28, 29, (30) |
| P | 15 | 31.04 | 1 | 31 |
| S. | 16 | 32.06 | 1 | 32 |
| Cl | 17 | 35.46 | 2 | 35, 37, (39) |
| A | 18 | 39.88 | 2 | 40, 36 |
| K | 19 | 39.10 | 2 | 39, 41 |
| Ni | 28 | 58.68 | 2 | 58, 60 |
| As | 33 | 74.96 | 1 | 75 |
| Br | 35 | 79.92 | 2 | 79, 81 |
| Kr | 36 | 82.92 | 6 | 84, 86, 82, 83, 80, 78 |
| Rb | 37 | 85.45 | 2 | 85, 87 |
| I | 53 | 126.92 | 1 | 127 |
| X | 54 | 130.2 | 5, (7) | 129, 132, 131, 134, 136, (128, 130 ?) |
| Cs | 55 | 132.81 | 1 | 133 |
| Hg | 80 | 200.6 | (6) | (197-200), 202, 204 |
Dempster's later results (V. p. 148)
| Ca | 20 | 40.07 | (2) | (40, 44, ?) |
| Zn | 30 | 65.37 | (4) | (64, 66, 68, 70) |
References
- ↑ Gehrcke and Reichenheim, Ver. d. Phys. Gesell, 8, 659, 1906; 9, 76, 200, 376, 1907; 10, 217, 1908.
- ↑ P, 31
- ↑ Dempster, Science, Dec, 10, 1920.[1]
- ↑ Dempster, Proc. Nat. Ac. Sci., 7, 45, 1921.[2]
- ↑ Aston, Phil. Mag., 42, 436, 1921.[3]
- ↑ Richardson, The Emission oj Electricity from Hot Bodies, p. 234 et seq., Longmans, 1916.[4]
- ↑ Dempster, Science, April 15, 1921.[5]
- ↑ V. Chap. V.
- ↑ G. P. Thomson, Phil. Mag., 42, 857, 1921.[6]
- ↑ V. p. 80.
- ↑ V. p. 86.
- ↑ V. p. 148.
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
